Solved) - NRT The Ideal Gas Equation States That Pi Where P Is The Pressure, (1 Answer)
amp;#160;NRT The Ideal Gas Equation States That Pi Where P Is The Pressure, N Is The V Number Of Moles Of Gas, R= .08206, T Is The Temperature (In Degrees Kelvin), And V Is The Volume Of The Gas. At High Pressure, A More Accurate Equation Is The Van NRT
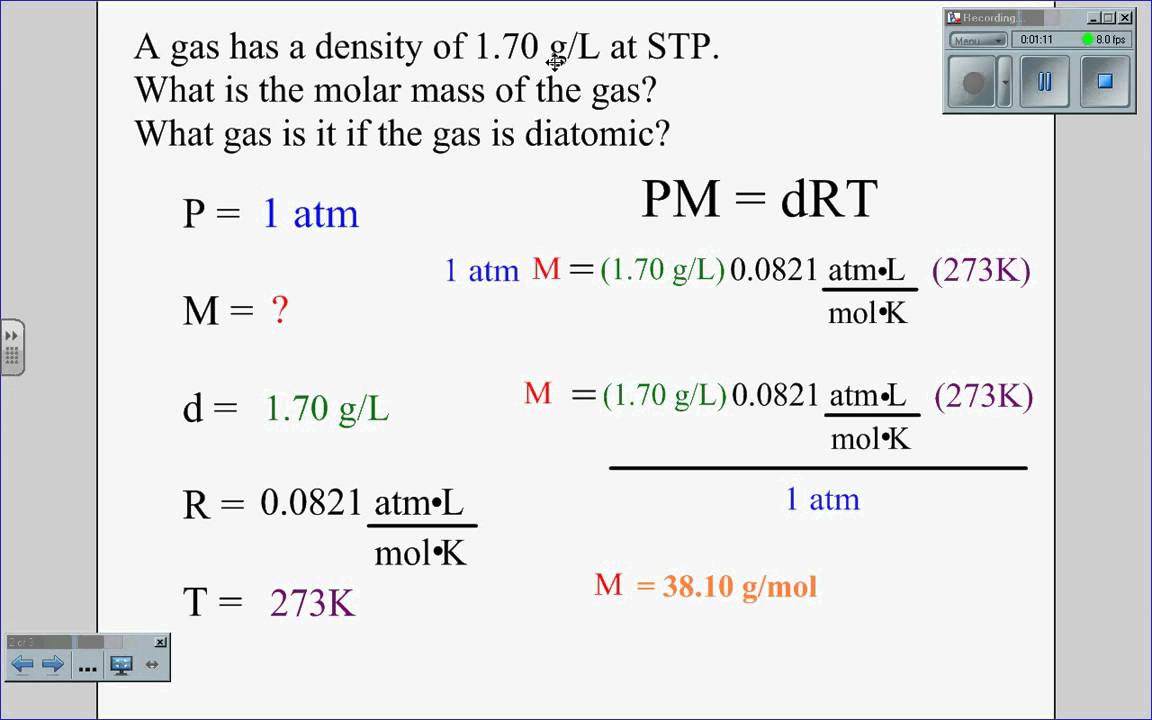
How do you solve ideal gas law problems?

NCERT Solutions for Class 11 Physics Chapter 13 Kinetic Theory.
The equation of state for real gas is given by (P+a/V2)(V b)=RT

Solved Question 6 NRT V The ideal gas equation states that P
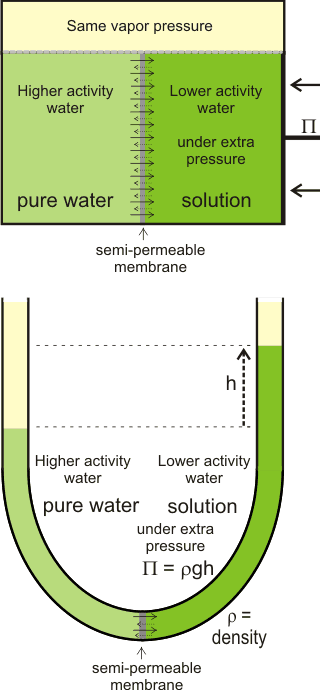
Osmotic pressure

An ideal gas initially at a state __(P1,V1)__ is allowed to expand isothermally to a state __(P2, V2)__. Then the gas is compressed adiabatically to its initial volume __V1__. Let the final
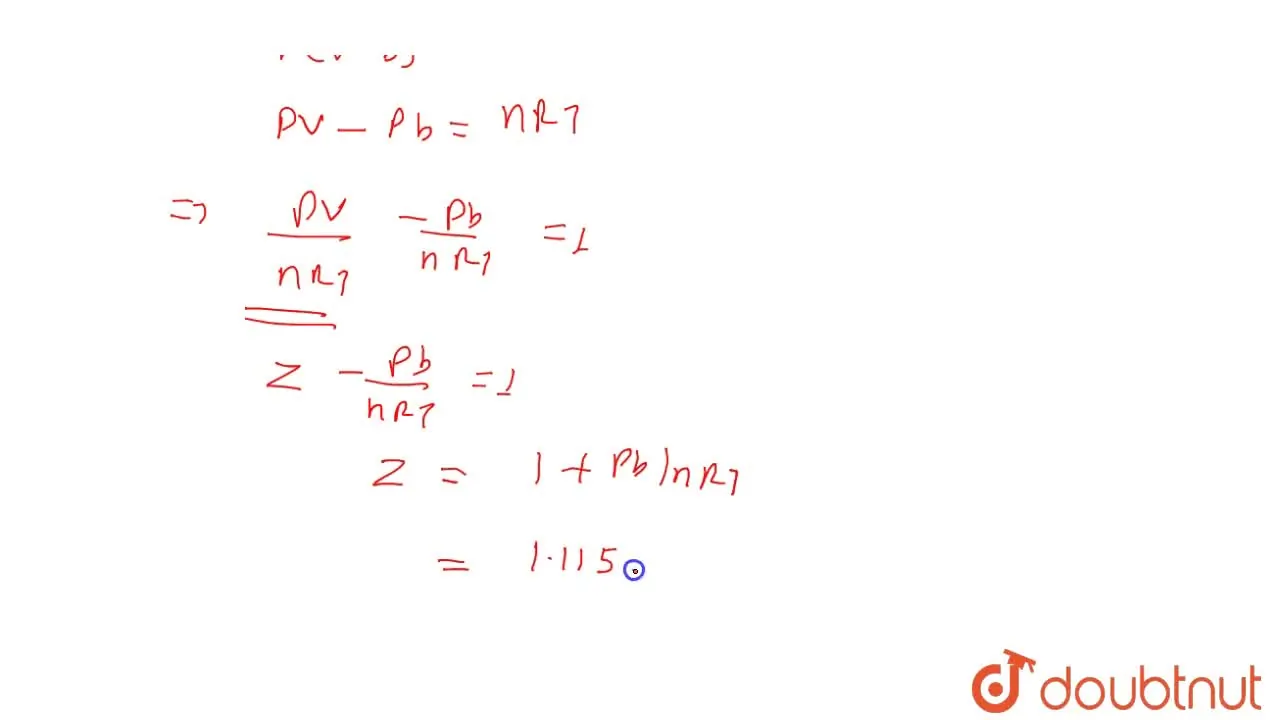
A real gas is supposed to obey the gas equation P(V-b) =nRT at STP if

Gas Laws - Equations and Formulas

The ideal gas equation states that: P = nRT/V where P is the

Let's Derive the Ideal Gas Law from Scratch!
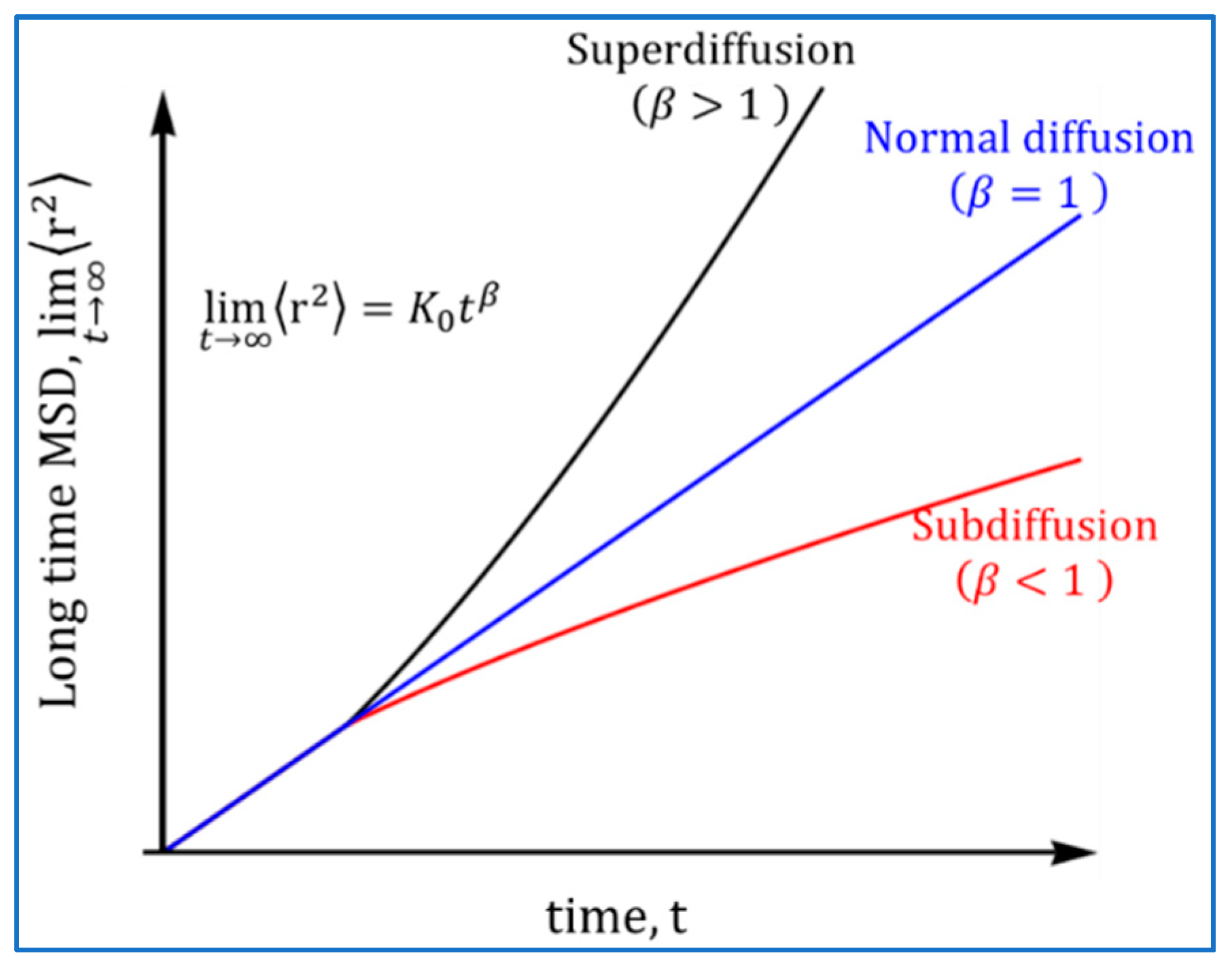
Processes, Free Full-Text

Common Chemistry Formulas and Equations Cheat Sheet
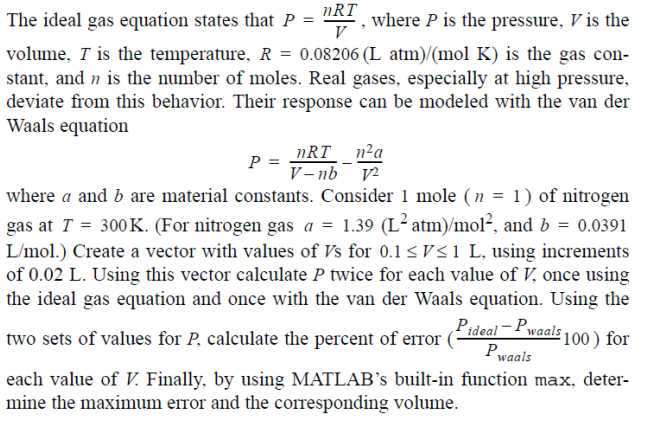
Solved nRT The ideal gas equation states that P = , where P









