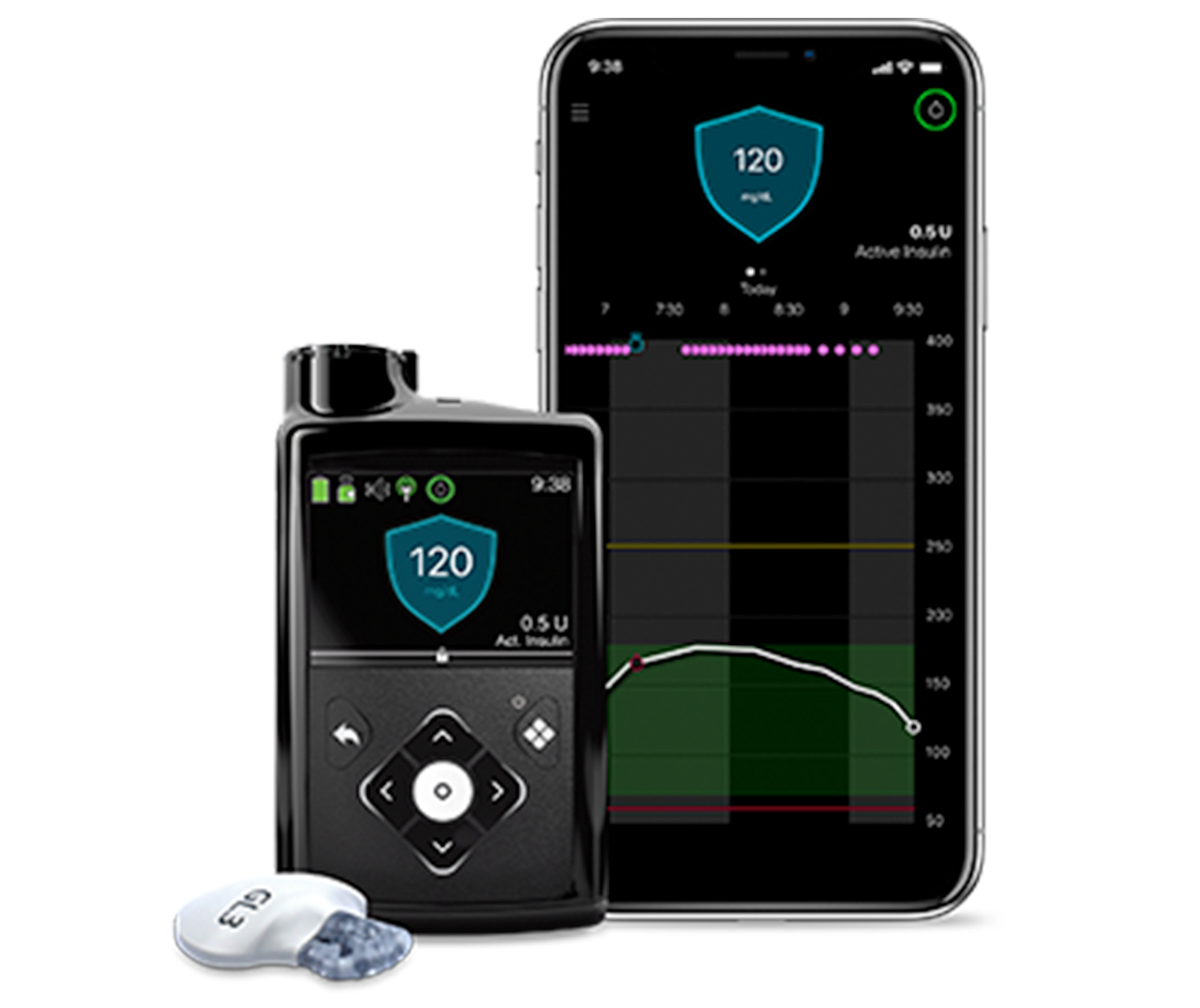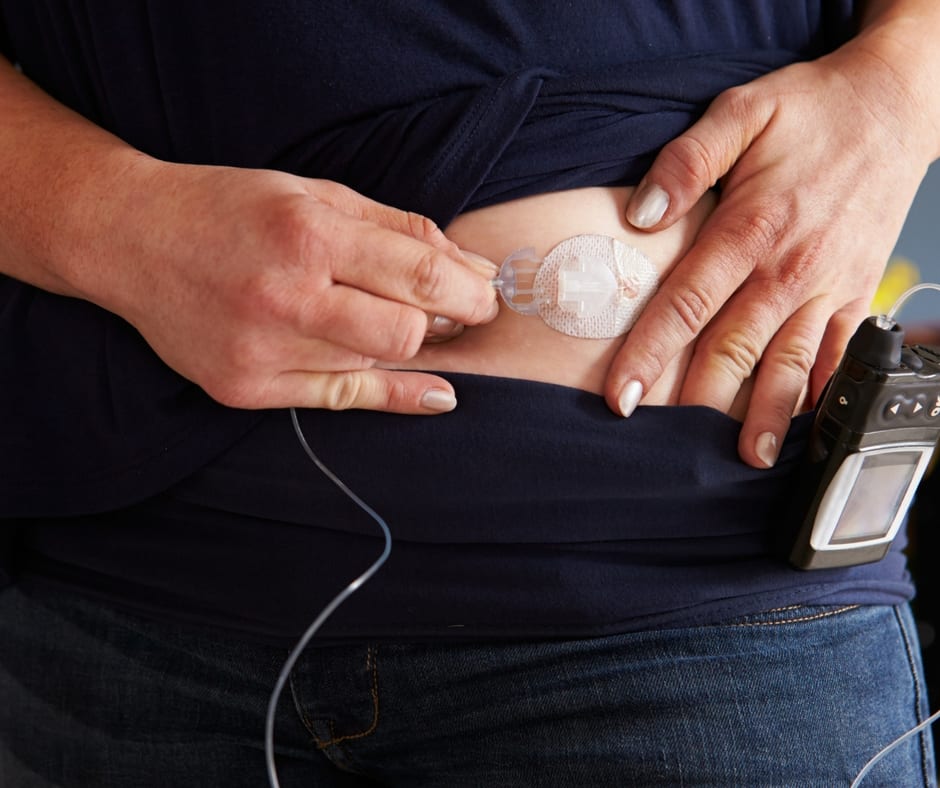
Understanding the Insulin Pump Recall
Medtronic is recalling defective MiniMed insulin pumps that could cause severe hypoglycemia or hyperglycemia resulting in seizures, coma and even death

FDA says Medtronic MiniMed insulin pump recall is serious - MassDevice

Medtronic Insulin Pumps - DeGaris Law, LLC
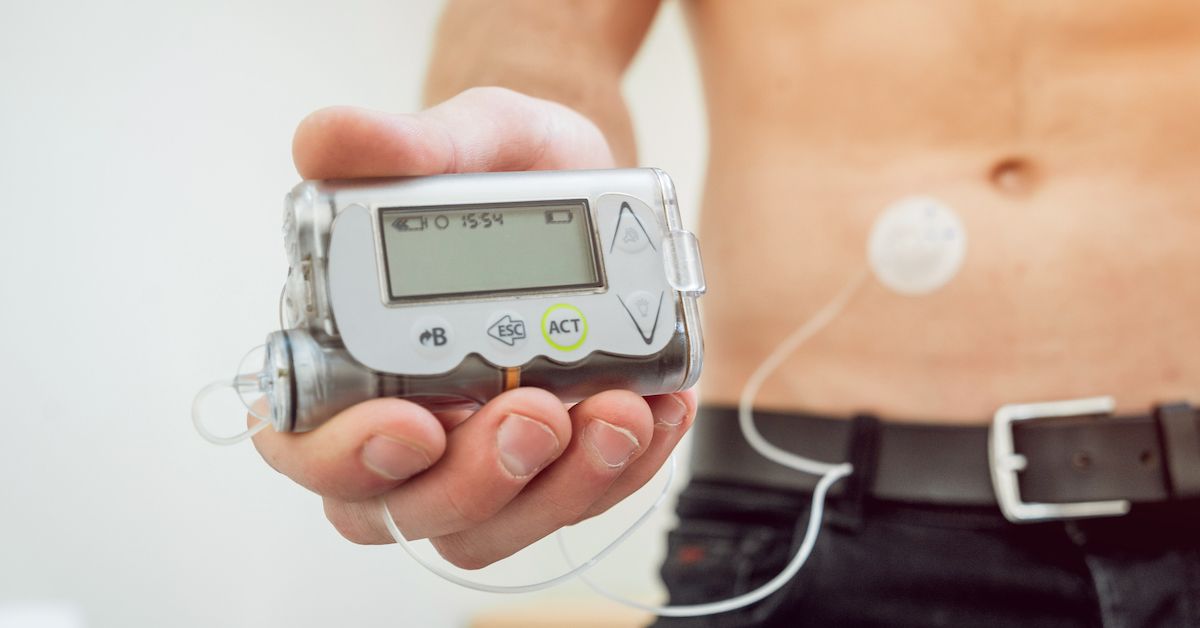
Hacking Risk Prompts Medtronic Recall of Insulin Pumps - Campus Safety

media-cldnry.s-/image/upload/t_nbcnews

AP Investigation: Insulin Pumps have high number of injuries

Fresenius Kabi has a Class I Ivenix infusion pump recall

Medtronic Diabetes on X: Medtronic Diabetes is proactively replacing all #MiniMed600 series clear retainer ring pumps with the same pump with a black ring, regardless of warranty status, free of charge. This

Medtronic Diabetes - *We're posting this to make sure everyone who needs this information gets it. If you are affected, please keep an eye on your email inbox, in addition to your

Insulin pumps recalled due to hacking risk

Understanding the Insulin Pump Recall